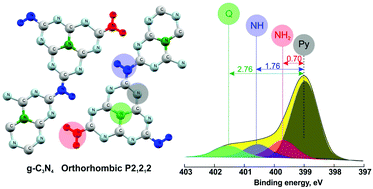
By using the most popular method of thermal condensation of dicyandiamide in a semi-closed system, graphitic carbon nitrides (gCNs) were synthesized at 500, 550, and 600 °C. The resulting materials were comprehensively analyzed via X-ray photoelectron spectroscopy (XPS) and X-ray powder diffraction (XRD)techniques. We show that the use of routine analytical methods provides an insight into the structure of the carbon nitride materials. The analysis of geometric linear structures and fully condensed structure of polymeric carbon nitrides was performed and the ranges within which the contents of different nitrogen species (pyridine, amine, imine and quaternary nitrogen) can change were determined. This analysis, in combination with quantitative XPS studies, permits to state that the carbon nitride structure prepared by the thermal condensation of dicyandiamide is closer to the structure in which poly(aminoimino)heptazine subunits are linked into chains rather than the structure involving fully-condensed polyheptazine network. The XRD analysis proved that the 3D crystal structure of carbon nitride is described more correctly by the orthorhombic cell and space group P21212 applied to condensed chains of poly(aminoimino)heptazine (melon) and not by the hexagonal cell with the space group P6m2.
Elucidating the structure of the graphitic carbon nitride nanomaterials via X-ray photoelectron spectroscopy and X-ray powder diffraction techniques
Dalton Transactions, vol. 49, pg. 12805-12813 (2020)