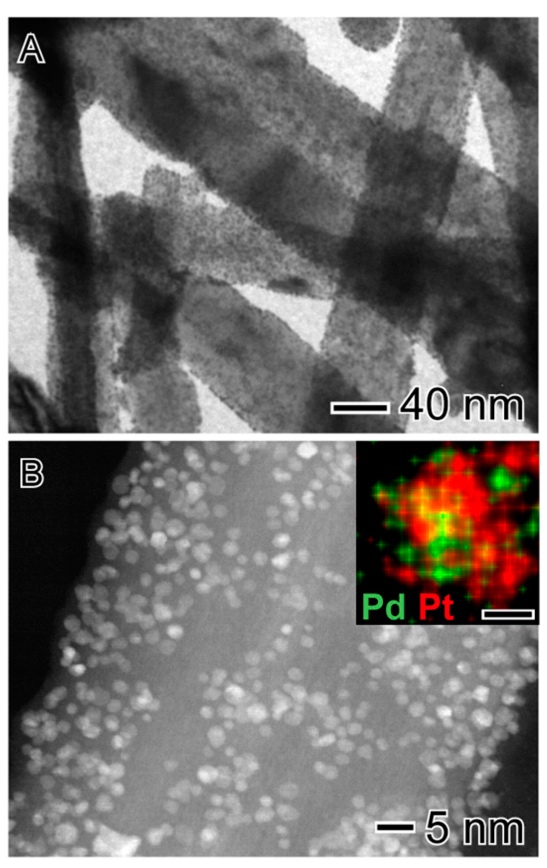
Mono and bimetallic catalysts based on Pt and Pd were prepared by a co-precipitation method. They were tested in liquid phase hydrogenation reactions of glucose and furfural at low temperature and pressure. The bimetallic PtPd/TiO2 catalyst proved to be an efficient material in selectively hydrogenating glucose to sorbitol. Moreover, high furfural conversion was attained under relatively soft conditions, and the furfuryl alcohol selectivity was strongly affected by the chemical composition of the catalysts. Furfuryl alcohol (FA) was the major product in most cases, along with side products such as methylfuran (MF), furan, and traces of tetrahydrofuran (THF). These results showed that the PtPd bimetallic sample was more active relative to the monometallic counterparts. A correlation between the catalytic results and the physicochemical properties of the supported nanoparticles identified key factors responsible for the synergetic behavior of the PtPd system. The high activity and selectivity were due to the formation of ultra-small particles, alloy formation, and the Pt-rich surface composition of the bimetallic particles supported on the TiO2 nanowires.
Exploiting the Synergetic Behavior of PtPd Bimetallic Catalysts in the Selective Hydrogenation of Glucose and Furfural
Catalysts, vol. 9, pg. 132 (2019)