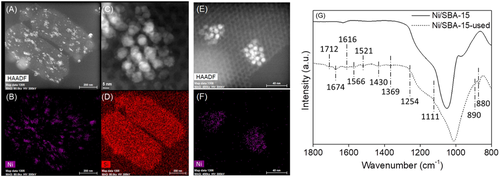
The selective hydroconversion of 5‐hydroxymethylfurfural (HMF) to biofuels is currently highly sought‐for. While the literature has demonstrated that this reaction is possible on promoted Ni catalysts, we show here that a monometallic, non‐promoted Ni/SBA‐15 catalyst, prepared by incipient wetness impregnation, can convert HMF to 2,5‐dimethylfuran (DMF) and to 2,5‐dimethyltetrahydrofuran (DMTHF) at 180 °C, in a consecutive way. Through a control over reaction time, high yields to DMF (71 %, at conversion of 93 %) or DMTHF (97 %, at conversion of 100 %) can be achieved. Kinetic modelling suggests a preferential route to DMF via 5‐methylfurfural (MFFR) as intermediate, though the route via 2,5‐bis(hydroxylmethyl)furan (BHMF) is also present. The favored route in the experimental conditions involves the hydrogenolysis of the hydroxyl group of HMF as first step, followed by the hydrogenation of the aldehyde function, to methylfurfuryl alcohol (MFOL). It is suggested a higher reaction rate of hydrogenation or hydrogenolysis of the side group is linked to the presence of a methyl group in the molecule. No hydrogenation of the furan ring is detected on the intermediates.
Hydroconversion of 5‐Hydroxymethylfurfural to 2,5‐Dimethylfuran and 2,5‐Dimethyltetrahydrofuran over Non‐promoted Ni/SBA‐15
ChemCatChem, vol. 12, pg. 2050-2059 (2020)